Spectroscopy in Art and Artifacts Authentication
Atomic physics has identified many famous forgeries
Cutting-edge science is used to identify, date, and authenticate many kinds of artifacts, from priceless paintings to prehistoric artifacts. This article shows how one advanced nuclear physics method developed by Physics Nobel Prize winners has been used to both authenticate and identify forgeries of some of the world’s most famous artifacts, from Vermeer paintings to Hitler’s Diaries.
In its most general sense, spectroscopy (often called spectrometry) is the science of examining and measuring light as it interacts with or is emitted by matter, and includes such basic things as measuring light passing through a prism and observing with our eyes the colors of objects. When you shine a blacklight on an object to see the color and brightness of the fluorescence, that is a basic form of spectroscopy.
In art and artifacts authentication and forgrery detection, however, spectroscopy involves various highly advanced methods of analyzing the molecular structure of material and objects by shining infrared, x-rays, gamma rays and lasers at the material and analyzing the electromagnetic radiation that is returned.
Whether reflected, fluoresced or scattered, the returned light is determined by the molecular makeup of the material, and the advanced forms of spectroscopy can be used to not only identify the material, but identify the material’s exact chemicals and compounds and their concentrations.
Knowing the material, chemicals and compounds is invaluable in authentication and forgery detection, and has identified some of the most sophisticated and famous forgeries. Many sophisticated forgeries have been identified because the chemicals and compounds identify the material as being from the wrong time and even originating from the wrong place. Spectroscopic analysis can go as far as identifying the geographical origins of pigments, ivory and gems.
.
Colorimetery: the scientific measuring visual light
While spectroscopy gets highly advanced and technical, a basic method of it is called colorimetry. Colorimetry measures the visual color of materials and objects.
The most basic form of colorimetery, and spectroscopy, is when we judge the color of something with our own eyes. Under white light, we see a ball as red or a coffee mug as blue. We identify different kinds of wood in part by their different shades of brown. The color of the light we see is determined by the atomic makeup of the material.
However, human vision is inexact and subjective. As demonstrated by color vision tests at the optometrist, it varies from person to person, and even a person’s eye to eye.
Colorimetry uses a scientific instrument called a colorimeter to measure color at a precise and objective level.
Identifying color at such a precise level is important in numerous areas and for many reasons, including when examining inks, paints, dyes and gems. In cases of court contested documents, such as wills and contracts, alterations to the writing are often discovered because a colorimeter identities by the color that different inks were used. The colorimeter identifies very slight differences in color of the inks that are unnoticeable by the naked eye.



.
Infrared, Raman, Mass and X-Ray Spectroscopy
As mentioned, advanced spectroscopy shines different ranges of electromagnetic radiation on the material and examines the light that is return. These methods use an expensive device called a spectrometer, which can be a stand alone, but is often hooked up to a computer and sometimes a microscope. They range in size from handheld to large complex-looking systems.
While there are many different kinds and variations of advanced spectroscopy used for many purposes and in many areas, the ones most commonly used to examine art and artifacts are infrared spectroscopy, Raman spectroscopy, X-ray fluorescence spectroscopy and mass spectrometry.
Infrared spectroscopy shines infrared light and measures the inter-atomic bond vibrations. It is based on that molecules absorb frequencies depended on their chemical structures.
Named after the 1930 Physics Nobel Prize winner C. V. Raman, Raman spectroscopy shines a laser beam of light, and measures slight energy changes to some of the scattered back light that is caused by material’s molecular vibrations. C. V. Raman was the first to publish a paper on this vibrational scattering, which is called Raman scattering or the Raman effect.
X-ray fluorescence spectroscopy measures the x-ray fluorescence given off from a material when shortwave x-rays or gamma rays are shined on the material. The shined x-rays or gamma rays add energy to the atoms. The atoms can hold this energy only for a short time before having to give it off. The atoms give off the energy in a different form than received– a longer wavelength of x-rays that is the fluorescence. You can see how this is related to ultraviolet or blacklight fluorescence, where the black light causes the material to give of a visible light fluorescence.
Done in a vacuum, mass spectrometry ionizes the atoms of the material and measures the mass-to-energy ratio. Francis Aston and J. J. Thompson won Physics Nobel Prizes for their work in this area.
These different types of spectroscopy examine and measure different aspects of the materials and create different spectrum charts. Shown on a computer screen, each spectrum is based on the molecular makeup of the material and and serves as a fingerprint for identifying the chemicals or compounds in the material. Each chemical or compound will have its own, unique spectrum.

The spectrometer has software that contains a library of the spectrums that will match up the tested material’s spectrum and tell you what what is the compound or chemical.
The process can be as simple as shining the spectrometer on the material, and the software telling you on the screen that the material is iodine, aspirin, gold or whatever it is. Handheld spectrometers are used at recycling centers to immediately identify the scrap metal compositions, and at airports to quickly identify mysterious substances, such as pills and powders.
Further, the height of the peaks of the on the spectrum tells you the concentration of the chemicals in the material.
Certain ranges of light interacts better with certain chemicals ,so the different types of spectroscopy are often used complementarily with each otherwhen examining a material. For example, infrared spectroscopy is better at reading a certain range of chemicals, while Raman spectroscopy a slightly different range. Thus, an old painting may be examined by both infrared and Raman spectrometers.
This analysis can be non-destructive— meaning no sample has to be removed from the object– and can often be be done on sight. The scientist can bring the Raman, infrared or x-ray fluorescence spectrometer to the huge painting on the wall of the museum, rather than the painting having to be brought to the lab.
The exception is with the mass spectrometer that requires a sample, in part because the process takes place in a vacuum.
.
Why being able to identify the chemicals and compounds is important to authentication and forgery detection
Knowledge of the materials and their chemical makeups in an artwork or artifact is important to authentication and forgery detection in many ways. There is much known, and continuous research, about the invention, chemical makeup and historical use and making of materials. It is sometimes even known where artists and cultures obtained the materials to make their objects– such as the imported minerals used to make paint or local stone to make artifacts. Thus, spectroscopic analysis of a questioned object can identify materials, chemicals and compounds in it that are consistent with the item being genuine and of the correct age, and conversely compounds or materials inconsistent if not impossible with the item being genuine. The following are examples:
Hans van Meegeren’s forgery of a 1600s Jan Vermeer painting was in part verified as fake because the paint contained Bakelite, a synthetic resin invented in the 20th century.
A painting forger used the correct type of lead white for an Old Master’s painting, but the specific compounds used to make the paint came from a geographical source unavailable to the original painter.
Forgeries of Man Ray’s photographs were identified due to too modern of chemicals in the photopaper.
Spectroscopy can tell the difference between natural and synthetic diamonds as it can identify the source chemicals the gems were produced from.
It has identified sophisticated forgeries of ancient precious metal relics, because, while the correct metal was used in the forgeries, the specific compounds of the metals were different than used by the original peoples.
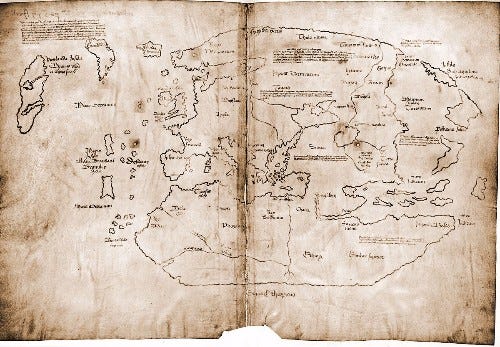
Spectroscopy identified the crystal anatase in the ink used on the Vinland Maps, with anatase being unknown in use that early.
The Hitler Diaries were identified as forgeries in part because the binding material was identified as a modern synthetic and the paper contained chemicals that were introduced after World War II.